By: Dr. Steven Benner and Nicole Willett
For many years, scientists have considered the model that life originated on Mars and was transported to Earth, rather than originating on Earth. This model turns on answers to the question: What molecular structures are necessary for biology to “switch on”, moving from an inanimate state to a living state, where reproduction and adaptation (key parts of Darwinian evolution) are able to allow life to manage challenges to its 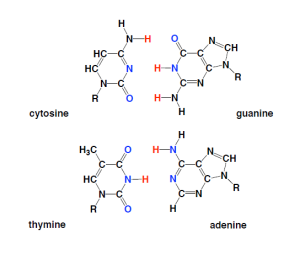 existence. For many, this switch requires the emergence, from a “prebiotic soup”, genetic molecules such as DNA and RNA. And, if this is true, the model then turns on the questions: Could genetic molecules have emerged on Earth? Could they have emerged on Mars? And given what we think about the environments on early Earth and Mars, which were more suited for the kinds of prebiotic chemistry that might give genetic molecules?
existence. For many, this switch requires the emergence, from a “prebiotic soup”, genetic molecules such as DNA and RNA. And, if this is true, the model then turns on the questions: Could genetic molecules have emerged on Earth? Could they have emerged on Mars? And given what we think about the environments on early Earth and Mars, which were more suited for the kinds of prebiotic chemistry that might give genetic molecules?
Dr. Steven Benner, of the Foundation for Applied Molecular Evolution in Florida, presented findings at the Goldschmidt Conference in Florence, Italy last week that suggest that Mars was more suited. His research increases the chance that life originated on Mars and was transported to Earth via meteorites. Some people say this is an outlandish claim, while others are becoming more intrigued by the facts that support this model.
To understand this subject, let’s start with some background information about chemistry and biology. Chemistry is the study of the elements (atoms) on the periodic table and how they connect and interact to make up everything in the universe, including you. Prebiotic chemistry is the study of how complex molecules that might allow the “switch” to biology might have emerged without life. Models in prebiotic chemistry describe how these non-biological molecules might, under defined conditions, somehow become biological. The missing link is the “somehow become biological”. Many studies and journal articles have been published on this subject. Some have been found to be incorrect and others linger with unanswered questions.
 The first form of life was, we presume, a single celled organism. Even so, the cells were complex compared to the prebiotic molecules that preceded them. The most important elements to early cells are, we presume, also those important to modern biology: carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. These were almost certainly combined on early Earth and Mars, first into small molecules (hydrogen cyanide, for example, HCN, or water HOH, or formaldehyde, HCHO). Processes are known where they can be further assembled (without life) to give components of genetic molecules, including the nucleic acid bases adenine, thymine, uracil, guanine, and cytosine. These bases are the individual letter codes commonly seen in articles and television shows where people check DNA tests.
The first form of life was, we presume, a single celled organism. Even so, the cells were complex compared to the prebiotic molecules that preceded them. The most important elements to early cells are, we presume, also those important to modern biology: carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. These were almost certainly combined on early Earth and Mars, first into small molecules (hydrogen cyanide, for example, HCN, or water HOH, or formaldehyde, HCHO). Processes are known where they can be further assembled (without life) to give components of genetic molecules, including the nucleic acid bases adenine, thymine, uracil, guanine, and cytosine. These bases are the individual letter codes commonly seen in articles and television shows where people check DNA tests.
But here the chemistry becomes more difficult. To further assemble these units into genetic molecules like RNA (believed to be a precursor of DNA), several things must happen. First, the organic molecules present on early Earth and early Mars, must avoid decomposition. As anyone knows who has left the stove on too long in the kitchen, organic molecules given energy tend to devolve into tar. For RNA to have a chance of emerging prebiotically, the devolution of its building blocks must be prevented.
In Florence, Benner presented evidence that minerals (like borax) containing the element boron (in the form of borate) are able to prevent this devolution. Borate captures carbohydrates that are formed in the prebiotic soup before they devolve to a tarry fate.
 Second, the atoms in the borate-captured carbohydrates must be rearranged to give ribose, the “R” in RNA. Dr. Benner presented the results of experiments that showed that minerals containing the element molybdenum (in its oxidized form, molybdate) can do this rearrangement.
Second, the atoms in the borate-captured carbohydrates must be rearranged to give ribose, the “R” in RNA. Dr. Benner presented the results of experiments that showed that minerals containing the element molybdenum (in its oxidized form, molybdate) can do this rearrangement.
Third, the ribose must be attached to adenine, uracil, cytosine, and guanine, each by forming bonds that are not easily formed in water. Then, phosphate must be added, also by forming bonds that are not stable in water. To do this, the amount of water available must be controlled; from time to time, the mixture must dry out.
This is all simple enough in the laboratory today. However, Dr. Benner pointed to models from geologists who hold that water was so abundant on early Earth that no dry land was available. Further, these models suggest that borate could not have been presented in useful concentrations. They also suggest that early Earth was insufficiently oxidizing to give molybdenum in its oxidized molybdate form. In short, geologists were suggesting that RNA could not have emerged on early Earth, at least not by way of the prebiotic chemistry that Dr. Benner has proposed.
 However, conditions on Mars appear to have been more favorable for Benner’s prebiotic chemistry. First, Mars has always had less water; it was easier to dry out on Mars. This should have allowed borate to be concentrated. Mars may have also had a more oxidizing environment, allowing for molybdate. Finally, phosphate may have been more accessible on early Mars.
However, conditions on Mars appear to have been more favorable for Benner’s prebiotic chemistry. First, Mars has always had less water; it was easier to dry out on Mars. This should have allowed borate to be concentrated. Mars may have also had a more oxidizing environment, allowing for molybdate. Finally, phosphate may have been more accessible on early Mars.
We know of this thanks to the orbiters, landers, and rovers that have been studying Mars for nearly 40 years. We have also collected a large number of meteorites that have come from Mars. These meteorites contain, among other things, borate minerals and other species that Benner’s prebiotic chemistry requires for the formation of RNA, which is believed to be a predecessor to DNA.
But even if Mars was a more suited planet for life to form, that life must have come to Earth. The idea that life is delivered to one planet from another is called panspermia. This is certainly possible. About one kilogram of Mars comes to Earth every day, after it is flung from Mars into the Solar System by a meteorite impacting on Mars. The low surface gravity of Mars makes escape from the Red Planet easier than from Earth. Reentry is sufficiently fast that microbes that originated on Mars would survive, arriving on Earth without damage. Here, they would find a planet that was habitable, able to sustain life, even Earth was not suited for life to originate in the first place.
 To further this analysis, we must fund and support missions to Mars that include new technology, such as the Icebreaker Mission. This mission has a six foot drill that will drill beneath the surface of Mars in order to get samples that are far enough below the surface to be shielded from harmful UV radiation. We must also fund and support missions that will send humans to Mars. We need humans on Mars in order to respond imaginatively to uncertain conditions on the planet, required to do the appropriate science with the proper laboratory equipment in order to get the answers that have eluded us for decades, possibly centuries. We need to find life on Mars in order to compare the DNA of the Martians to the DNA of the Earthlings. Could we all be Martians?
To further this analysis, we must fund and support missions to Mars that include new technology, such as the Icebreaker Mission. This mission has a six foot drill that will drill beneath the surface of Mars in order to get samples that are far enough below the surface to be shielded from harmful UV radiation. We must also fund and support missions that will send humans to Mars. We need humans on Mars in order to respond imaginatively to uncertain conditions on the planet, required to do the appropriate science with the proper laboratory equipment in order to get the answers that have eluded us for decades, possibly centuries. We need to find life on Mars in order to compare the DNA of the Martians to the DNA of the Earthlings. Could we all be Martians?
“The emergence of life on Earth might have been an inevitable consequence of the laws of physics, and if that is true, then a living cosmos might be the only way our cosmos can be” [Professor Brian Cox]
[Images: Benner, FSU, Md Weather, Spaceports, NASA]